Diamond has exceptional properties. So far known it is the hardest material on earth and the best heat conductor. Moreover, it has a good chemical resistance and the highest breaking index of all natural materials. Due to these unique characteristics diamond has also special applications. The best known are jewelries; the high breaking index gives a special sparkling effect. Most diamonds are used in technical applications like chisels, heatsinks, high pressure anvils, windows and surgical cutting tool.
Source: Diamond research of the Department of Solid State Physics II; John Schermer
Images: Electron microscopy of diamond crystals
| Diamond growth |
 Since it is known that diamond is formed out of carbon, attempts ahve been made to convert cheap forms of carbon into diamond. Meanwhiles a number of methods have be developed for this purpose. One of these methods employs a simple oxygen-acetylene burner. In the acetyle flame carbon hydrogen radicals are built which then deposits on a substrate so that diamond crystals can gradually grow. Since it is known that diamond is formed out of carbon, attempts ahve been made to convert cheap forms of carbon into diamond. Meanwhiles a number of methods have be developed for this purpose. One of these methods employs a simple oxygen-acetylene burner. In the acetyle flame carbon hydrogen radicals are built which then deposits on a substrate so that diamond crystals can gradually grow.
|
| Diamond crystals |
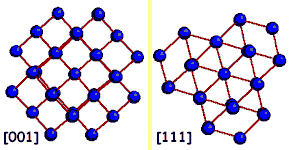 |
A crystal is a periodical regularly ordered, repeating pattern extending in all three spatial dimensions. Through a coordinate system orientations and surfaces in crystals can be easily defined. The arrangement of the atoms at the surface is determinant for the way in which the crystal grows. Normally, a diamond crystal is only bordered by stable {001} and {111} surfaces. These smooth surfaces, called also faces, are in fact a interception surfaces through the diamond crystal. |
 |
The arrangement of atoms at the surface of these faces varies clearly with the cleavage surface (the zoom in animation shows how the arrangement of atoms changes from a quartet into a triplet symmetry when the crystal is rotated from the <001> direction to the <111> direction). This has as a consequence that the growth of diamond evolves differently on the {111} faces than on the {001} faces.
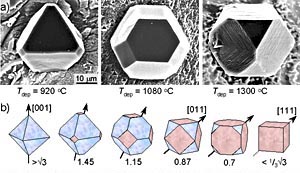
The shape of a perfect diamond crystal is influenced by the growth conditions (like temperature) that cause variations in growth rate on the and faces (a). Reversiverly, the relative growth rates in the single crystallographic directions can be determined from the observed crystals shapes. The range of possible cubo-octaeder crystalas conssiting of {001} and {111} faces (the red respectively blue planes) is schematically represented in (b). The ratio of the growth rates in either direction V[001]/V[111] has been given under each crystal structure. The arrows indicate the direction with the greatest growth rate: the corresponding faces grow from the final crystal structure which is determined by the most slowly growing faces.
|
| Microscopical errors in the crystal structure |
 | In practice the growth proces seldomly occurs perfectly and errors occurs often in the crystallization pattern depending on the growth conditions. An important class of defects arise fromthe fact that a new crystal is formed on an already existing crystal. In this way it becomes more and more difficult to recognize the original crystalline structure (a). A special kind of this secondary nucleation process is the one generating twin crystals (b). More on crystallographic defects in diamond... |
| Diamond layers |
 | During the growth process crystals become so large after a while that they build a continuous poly-crystalline diamond layer in which crystals press each other aside (see FESEM simulation here above in a high magnification view of a poly-crystalline layer ). |
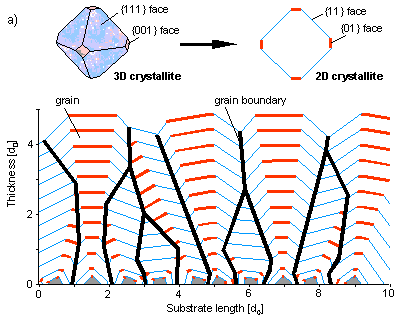 |
In case the secondary nucleation is suppressed each of these crystals has been initiated at the beginning of the primary process on the substrate, and the course of growth can be easily simulated in two dimensions (a) (start simulation).
 This clearly shows that crystals oriented by chances with the most rapidly growing face perpendicular to the substrate will survive in the end. In contrast tot the situation for an individual crystal, here it is the most rapidly growing crystal that is determinant for the morphology of the poly-crytalline layer (b). In practice it appears to be very difficult to suppress secondary nucleation. In general we find a layer with strongly distorted crystals in which and faces are hard to discrimate from another.
This clearly shows that crystals oriented by chances with the most rapidly growing face perpendicular to the substrate will survive in the end. In contrast tot the situation for an individual crystal, here it is the most rapidly growing crystal that is determinant for the morphology of the poly-crytalline layer (b). In practice it appears to be very difficult to suppress secondary nucleation. In general we find a layer with strongly distorted crystals in which and faces are hard to discrimate from another.
|
| Physics research on crystals involving the FESEM |
|  |
 |

|
| Zoom of this stereo view on crystals (click on the thumbnail to get a zoomed image; depth can be seen by looking though stereo glasses consisting of a red filter for one eye and a green filter for the other eye; tip: cut filters from transparent projection sheets) | Short paper in Dutch on thin layers of poly-crystalline materials that are investigated for possible applications in the micro-chip industry (229 KB in pdf in Dutch) | PhD thesis of M. Moret (in English) |








